Abstract
Background: CD19 targeting chimeric antigen receptor (CAR) T cells have induced unprecedented remission rates in high-risk precursor B Acute Lymphoblastic Leukemia (ALL); however recurrent disease with CD19 antigen escape variants is not uncommon. Therefore, we developed a novel CD22 targeting CAR, and following preclinical validation, tested it in a first-in-human pediatric and young adult phase 1 clinical trial, PLAT-04 (NCT03244306). Four subjects were treated at 2 dose levels (DL) (1x10 6/kg (DL1) and 3x10 6/kg (DL2)). The CD22 CAR T cell product (SCRI-CAR22v1) was successfully manufactured (n=4) and no dose limiting toxicity (DLTs), cytokine release syndrome (CRS) or neurotoxicity was observed. However, all subjects had minimal CAR T cell expansion, with 3 of 4 subjects demonstrating persistent or progressive disease at day 21 evaluation despite continued CD22 expression on leukemic blasts. Based on the poor in vivo expansion and lack of activity, enrollment was voluntarily halted to interrogate and optimize the CAR construct for enhanced performance.
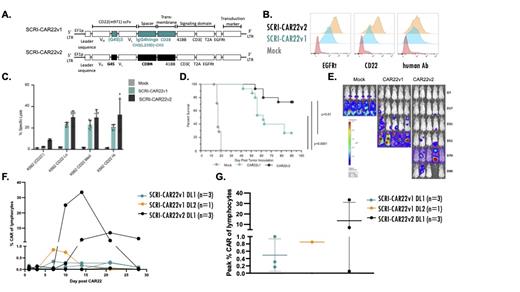
Methods: Human T cells were transduced to express one of two CD22 CAR constructs. We designed SCRI-CAR22v2, a CD22 CAR that utilizes the same scFv as SCRI-CAR22v1 but with a shorter linker between M971 VH and VL and a shorter hinge with differing transmembrane region, and both using CD8 alpha (Figure A). This construct maintained the truncated EGFR extracellular tag (EGFRt) for tracking and potential in vivo suicide mechanism. The two transduced CAR T cell products were compared preclinically by flow cytometry, chromium release assay and in an in vivo murine model to understand differing T cell activity between the CAR constructs. Additionally, SCRI-CAR22v2 is currently under investigation in a dose finding phase 1 clinical trial, PLAT-07 (NCT04571138).
Results: Following use of cetuximab-APC and biotinylated anti-human Fab antibody for surface EGFRt and CAR detection, the SCRI-CAR22v1 expresses lower levels of EGFRt but similar CAR levels on the cell surface demonstrated by MFI (Figure B). Biotinylated, soluble CD22 antigen was also used to evaluate CD22 CAR receptor activity and, as measured by MFI, a higher affinity is suggested via SCRI-CAR22v2 as compared to SCRI-CAR22v1 (Figure B). K562 cells expressing low, medium or high CD22 were used to evaluate the impact of surface antigen expression on the CAR activity level. SCRI-CAR22v2 demonstrates improved targeted cell lysis at all 3 antigen quantity levels by chromium release assay (Figure C). In NSG mice inoculated with Raji tumor cells expressing ffluc, SCRI-CAR22v2 demonstrated improved survival compared to SCRI-CAR22v1 (Figure D) and clearance of Raji tumor cells (Figure E). Based on this promising preclinical data, we initiated enrollment onto PLAT-07, a phase 1 dose finding trial (2x10 5cells/kg (DL1), 5x10 5cells/kg (DL2) and 1x10 6cells/kg (DL3)) of SCRI-CAR22v2. To date, 3 subjects have been enrolled and successfully infused at DL1. All had prior CD19-CAR therapy and 2 lacked CD19 leukemic expression at the time of SCRI-CAR22v2 infusion. At the time of cell infusion, one subject had only extramedullary disease, one had MRD of <1% and one subject had a larger disease burden of 30% ALL. None experienced a DLT and all were MRD negative in the bone marrow at day 28 and the subject with EMD demonstrated a complete metabolic response by PET scan. Figure F exhibits the improved expansion and engraftment of the SCRI-CAR22v2 cells as compared to SCRI-CAR22v1 DL1 (n=3) and DL2 (n=1), and higher peak levels of CD22 CAR T cells as compared to SCRI-CAR22v1 DL1 and DL2 (Figure G).
Conclusions: Despite encouraging preclinical data, SCRI-CAR22v1 demonstrated poor expansion and engraftment in a Phase 1 trial. Notably, minor CAR alterations lead to encouraging in-human activity in early clinical findings. Our experience suggests a shorter linker and hinge as well as incorporation of an CD8 alpha transmembrane region improves the clinical activity of CD22 targeted CAR T cells in subjects with recurrent disease following CD19 CAR T cells. Further evaluation is needed to elucidate the critical CAR components and/or assays at the preclinical level that can best predict which CAR should be brought to the clinic for further evaluation.
Orentas: Lentigen: Patents & Royalties. Jensen: BMS: Patents & Royalties; Umoja Biopharma: Current holder of stock options in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Research Funding; Bluebird Bio: Research Funding. Gardner: Novartis: Consultancy; BMS: Patents & Royalties.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal